UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
FORM 6-K
REPORT OF FOREIGN
PRIVATE ISSUER PURSUANT TO RULE 13a-16 OR 15d-16
UNDER THE SECURITIES EXCHANGE ACT OF 1934
July 15, 2021
Commission File Number: 001-39363
IMMATICS N.V.
Paul-Ehrlich-Straße 15
72076 Tübingen, Federal Republic of Germany
(Address of principal executive office)
Indicate by check mark whether the registrant files or will file annual reports under cover of Form 20-F or Form 40-F:
| Form 20-F |
☒ |
Form 40-F |
☐ |
Indicate by check mark if the registrant is submitting the Form 6-K in paper as permitted by Regulation S-T Rule 101(b)(1): ☐
Indicate by check mark if the registrant is submitting the Form 6-K in paper as permitted by Regulation S-T Rule 101(b)(7): ☐
INFORMATION CONTAINED IN THIS REPORT ON FORM 6-K
On July 15, 2021, Immatics N.V. (the “Company”) made available an updated investor presentation on its website. A copy the investor presentation is attached hereto as Exhibit 99.1. The fact that this presentation is being made available and filed herewith is not an admission as to the materiality of any information contained in the presentation. The information contained in the presentation is being provided as of July 15, 2021 and the Company does not undertake any obligation to update the presentation in the future or to update forward-looking statements to reflect subsequent actual results.
EXHIBIT INDEX
| Exhibit No. | Description |
| 99.1 | Investor presentation dated July 2021 |
SIGNATURES
Pursuant to the requirements of the Securities Exchange Act of 1934, the registrant has duly caused this report to be signed on its behalf by the undersigned, thereunto duly authorized.
| IMMATICS N.V. | ||
| Date: July 15, 2021 | ||
| By: | /s/ Harpreet Singh | |
| Name: | Harpreet Singh | |
| Title: | Chief Executive Officer | |
Exhibit 99.1

© Immatics. Not for further reproduction or distribution. Unlocking Immunotherapies for Solid Cancer Patients Immatics Corporate Presentation, July 2021

Forward - Looking Statements 2 This presentation (“Presentation”) is provided by Immatics N . V . (“Immatics” or the “Company”) for informational purposes only . The information contained herein does not purport to be all - inclusive and Immatics nor any of its affiliates nor any of its or their control persons, officers, directors, employees or representatives makes any representation or warranty, express or implied, as to the accuracy, completeness or reliability of the information contained in this Presentation . You should consult your own counsel and tax and financial advisors as to legal and related matters concerning the matters described herein, and, by accepting this presentation, you confirm that you are not relying upon the information contained herein to make any decision . Forward - Looking Statements . Certain statements in this presentation may be considered forward - looking statements . Forward - looking statements generally relate to future events or the Company’s future financial or operating performance . For example, statements concerning timing of data read - outs for product candidates, the clinical trial application for IMA 204 , IMA 301 , IMA 401 , the Company’s focus on partnerships to advance its strategy, projections of future cash on hand and other metrics are forward - looking statements . In some cases, you can identify forward - looking statements by terminology such as “may”, “should”, “expect”, “intend”, “will”, “estimate”, “anticipate”, “believe”, “predict”, “potential” or “continue”, or the negatives of these terms or variations of them or similar terminology . Such forward - looking statements are subject to risks, uncertainties, and other factors which could cause actual results to differ materially from those expressed or implied by such forward looking statements . These forward - looking statements are based upon estimates and assumptions that, while considered reasonable Immatics and its management, are inherently uncertain . New risks and uncertainties may emerge from time to time, and it is not possible to predict all risks and uncertainties . Factors that may cause actual results to differ materially from current expectations include, but are not limited to, various factors beyond management's control including general economic conditions and other risks, uncertainties and factors set forth in the Company’s filings with the Securities and Exchange Commission (SEC) . Nothing in this presentation should be regarded as a representation by any person that the forward - looking statements set forth herein will be achieved or that any of the contemplated results of such forward - looking statements will be achieved . You should not place undue reliance on forward - looking statements, which speak only as of the date they are made . Company undertakes no duty to update these forward - looking statements . No Offer or Solicitation . This communication is for informational purposes only and does not constitute, or form a part of, an offer to sell or the solicitation of an offer to sell or an offer to buy or the solicitation of an offer to buy any securities, and there shall be no sale of securities, in any jurisdiction in which such offer, solicitation or sale would be unlawful prior to registration or qualification under the securities laws of any such jurisdiction . No offer of securities shall be made except by means of a prospectus meeting the requirements of Section 10 of the Securities Act of 1933 , as amended, and otherwise in accordance with applicable law . Certain information contained in this Presentation relates to or is based on studies, publications, surveys and the Company’s own internal estimates and research . In addition, all of the market data included in this presentation involves a number of assumptions and limitations, and there can be no guarantee as to the accuracy or reliability of such assumptions . Finally, while the Company believes its internal research is reliable, such research has not been verified by any independent source . Clinical study results and associated biomarker studies presented within this presentation are by definition prior to completion of the clinical trial and a clinical study report and, are therefore, preliminary in nature and subject to further quality checks including customary source data verification . This meeting and any information communicated at this meeting are strictly confidential and should not be discussed outside your organization .

Building a leading TCR Therapeutics Company with a Pipeline in Cell Therapies and Bispecifics 3 Highly Differentiated Technologies to Identify True Cancer Targets and the Right TCRs Unlocking Immunotherapies for Solid Cancer Patients Strategic Collaborations with World - leading Industry Players

Limitations of Current Immunotherapies in Solid Cancer Patients … Driven by a Lack of Known Cancer - specific Targets 4 1 Chalmers et al. , 2017; 2 SEER Cancer Statistics Review, 1975 - 2017, Estimated New Cancer Cases for 2020 Checkpoint inhibitors mainly effective in tumors with high mutational burden minority of all cancers 1 CAR - T mainly effective in hematological malignancies minority of all cancers 2 Most cancer patients do not benefit from current immuno - oncology approaches Intro We are unlocking immunotherapies for solid cancer patients with high unmet medical need by accessing intracellular cancer targets with TCR - based therapeutics Solid tumors limited established treatments & high medical need majority of all cancers
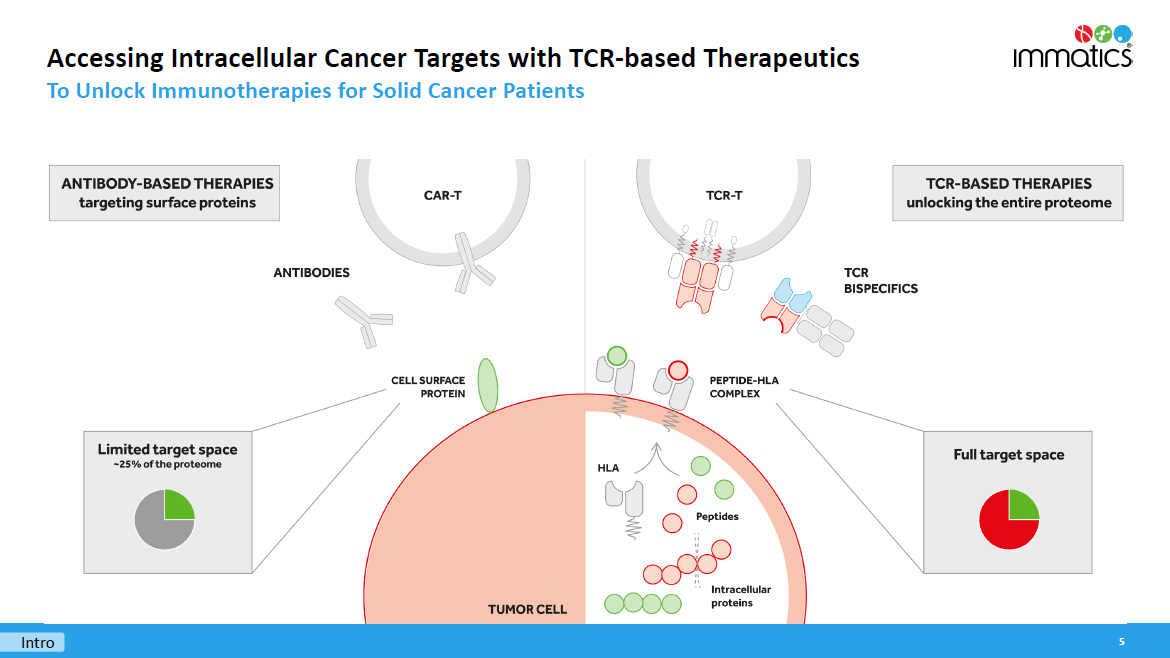
Accessing Intracellular Cancer Targets with TCR - based Therapeutics To Unlock Immunotherapies for Solid Cancer Patients 5 Intro

Immatics’ Targeted Approach in Two Distinct Therapeutic Modalities 6 High tumor burden Lower tumor burden 1 1 Patients at earlier stage of disease, de - bulked tumors, or late - stage patients with reduced tumor burden Specialized Centers All Hospitals & Out - Patient Intro

The Immatics Approach to Disrupt Current Tumor Treatment Paradigms Based on 5 Defined Principles 7 1. True Cancer Targets & Matching Right TCRs 2. Targeted Approach in Two Distinct Modalities: Adoptive Cell Therapy & TCR Bispecifics 3. Optimized Manufacturing to Enhance T cell Persistence & Efficacy 4. Disrupting the Tumor Microenvironment by Targeting Stroma 5. Combating Tumor Heterogeneity & Escape through Multi - Target Approach Intro

Immatics ’ Pipeline 8 Modality Product Candidate Status Preclinical Phase 1a 1 Phase 1b 1 Phase 2 Phase 3 Autologous ACT ACTengine® IMA201 (MAGEA4/8) Proprietary ACTengine® IMA202 (MAGEA1) Proprietary ACTengine ® IMA203 (PRAME) Proprietary ACTengine® IMA204 (COL6A3) Proprietary ACT programs (Undisclosed) ACT programs (Undisclosed) Allogeneic ACT ACTallo® IMA301 (Undisclosed) Proprietary Bispecifics TCER® IMA401 (MAGEA4/8) Proprietary TCER® IMA402 (PRAME) Proprietary Bispecific programs (Undisclosed) Bispecific programs (Undisclosed) 1 Phase 1a: Dose escalation, Phase 1b: Dose expansion Intro

Immatics’ Pipeline Addresses Significant Number of Cancer Patients Prevalence of MAGE4/8, MAGEA1 and PRAME in Major Solid Cancer Indications 9 Niche Indications with Fast Track Development Potential Squamous NSCLC HNSCC ~23,500 HLA - A*02 + cases p.a. Uterine Carcinoma Ovarian Cancer 35% 50% ~23,500 HLA - A*02 + cases p.a. ~27,000 HLA - A*02 + cases p.a. Synovial Sarcoma (e.g. PRAME prevalence 100%) and other Sarcoma subtypes ~9,000 HLA - A*02 + cases p.a. Bladder Cancer ~33,500 HLA - A*02 + cases p.a. 30% 20% Esophageal Cancer ~7,500 HLA - A*02 + cases p.a. 25% 20% 35% 65% 25% 20% Hepatocellular Carcinoma 40% 80% ~13,000 HLA - A*02 + cases p.a. Melanoma ~41,000 HLA - A*02 + cases p.a. 30% 95% 100% Breast Carcinoma ~113,000 HLA - A*02 + cases p.a. 25% MAGEA4/8 MAGEA1 PRAME Uterine Carcinosarcoma Cholangiocarinoma Anal Squamous Cell Cancer Selected tumor indications, prevalences based on IMADetect ® threshold; Based on HLA - A*02 prevalence in the US population (41%), https://seer.cancer.gov and internal market research IMA201/IMA401 IMA202 IMA203 Intro

Adoptive Cell Therapy 10

IMA201 IMA202 IMA203 Peptide Target HLA - A*02 - presented peptide derived from MAGEA4/8 MAGEA1 PRAME shown to be naturally and specifically presented on native tumor tissues at d ifferentiated high peptide target density 1 100 - 1,000 copies/cell 50 - 900 copies/cell 100 - 1,000 copies/cell T cell Receptor (TCR) High - affinity specific TCRs with high functional avidity 2 Natural TCR ~10 ng/ml Natural TCR ~15 ng/ml Pairing - enhanced TCR ~5 ng/ml T cell Product Autologous T cells gene - engineered with lentiviral vector expressing TCR and applying proprietary short - term manufacturing process designed to achieve better T cell engraftment and persistence 7 - 10 days 3 7 - 10 days 3 6 - 7 days 3 Key Features of Our Clinical ACTengine ® Programs 11 1 Applying XPRESIDENT® quantitative mass spectrometry engine; target density: p eptide copy number per tumor cell, approximate range representing the majority of tumor samples analyzed (25 - 75% percentiles) 2 Applying XCEPTOR® TCR discovery and engineering platform; functional a vidity : EC50 half maximal effective concentration, 3 Considering activation, transduction and expansion, subject to duration for release testing Differentiated Targets, TCRs and Cellular M anufacturing D esigne d to Enhance Safety and Activity ACT

ACTengine ® Clinical Programs – Clinical Overview & Patient Flow 12 * Thereof 10 patients evaluable for biological activity and clinical efficacy analysis at data cut - off; * * D ose modifications of lymphodepletion regimen for certain risk groups (e.g. patients with HCC & patients with reduced renal - clearance ). High Enrollment Efficiency through Combined Screening for Three Targets Leuka - pheresis HLA - A*02 Testing Blood sample; Central lab ACTengine ® Manufacturing by Immatics Infusion of ACTengine® T cell Product Lymphodepletion 40 mg/m 2 Flu darabine and 500 mg/m 2 Cy clophosphamide for 4 days ** Target Profiling Fresh Tumor Biopsy; IMADetect® IL - 2 1m IU twice daily for 14 days IMA201 IMA202 IMA203 14 Patients infused across three TCR - T Programs, as of data cut - off on Feb 16, 2021 * <1bn T cells infused per patient at dose levels 1 and 2 – presumed to be sub - therapeutic ACT

13 TEAEs by maximum severity ( N=16) Adverse event All Grades ≥ Grade 3 No . % No . % Patients with any adverse event 16 100.0 16 100.0 Lymphopenia 16 100.0 16 100.0 Leukopenia 16 100.0 16 100.0 Neutropenia 16 100.0 15 93.8 Anaemia 16 100.0 10 62.5 Thrombocytopenia 15 93.8 6 37.5 Nausea 11 68.8 0 0 Pyrexia 8 50.0 0 0 Vomiting 6 37.5 1 6.3 Fatigue 5 31.3 1 6.3 Hypoxia 5 31.3 1 6.3 Hyponatraemia 5 31.3 0 0 Dyspnoea 1 3 18.8 1 6.3 Atrial fibrillation 2 12.5 1 6.3 Hypertension 2 12.5 1 6.3 Muscular weakness 2 12.5 1 6.3 Pleural effusion 2 12.5 1 6.3 Tumor pain 2 12.5 1 6.3 Blood alkaline phosphatase increased 1 6.3 1 6.3 Candida infection 1 6.3 1 6.3 Corona virus infection 1 6.3 1 6.3 Febrile neutropenia 1 6.3 1 6.3 Infection 1 6.3 1 6.3 Pneumonia 1 1 6.3 1 6.3 Sepsis 2 1 6.3 1 6.3 Adverse Events of Special Interest Cytokine release syndrome 3 13 8 1.3 0 0 ICANS 4 3 18.8 0 0 All treatment - emergent adverse events (TEAEs) with grade 1 - 2 occurring in at least 5 patients (incidence ≥ 31 . 3 % ) and additionally all events with grade 3 - 5 regardless of relatedness to study treatment are presented . Data source : clinical and safety database ; h ematological adverse events were derived from lab values . Grades were determined according to National Cancer Institute Common Terminology Criteria of Adverse Events, version 5 . 0 . Grades for CRS and ICANS were determined according to CARTOX criteria ( Neelapu et al, 2018 ) . Patients are counted only once per adverse event and severity classification . 1 Patient died from tumor progression and pneumonia 69 days after IMA 202 T cell infusion (determined not related to any study medication), 2 Patient died from sepsis of unknown origin and did not receive IMA 203 T cells, 3 CRS : Cytokine release syndrome, 4 ICANS : Immune effector cell associated neurotoxicity syndrome, 5 DLT : Dose limiting toxicities Adverse Events: • Most frequent adverse events were transient cytopenia s associated with lymphodepletion • Transient CRS 3 (Grade 1 - 2) in 13/14 infused patients. • Transient G rade 1 or 2 ICANS in 3/14 infused patients , resolved wi th in 48 h in all cases Dose - limiting toxicities: • IMA201 and IMA202: No DLT 5 observed • IMA203: One transient, Grade 3 atrial fibrillation with onset on day 5 post infusion that resolved within 48h after onset. DLT triggered expansion of dose level 2 from three to six patients Data cut - off – February 16, 2021 ACTengine® Clinical Programs – Safety Profile Treatment - emergent Adverse Event s Are Manageable, Transient and Expected for Cell Therapies ACT

ACTengine® Clinical Programs – Biological Activity T cells R obustly Engraft, Persist and Infiltrate into Tumor after Infusion of Low Doses of ACTengine® • Robust T cell engraftment and persistence post infusion until the end of the observation period as assessed by qPCR * • Engineered T cells are detectable in serial tumor biopsies post T cell infusion in all evaluable patients by qPCR 14 Engraftment & T cell Persistence in the Blood Detection of T cells in the Tumor * Up to 9 month s (data not shown), UD: Undetected, NA: Not available, DL: Dose level, EC1: Enrichment cohort with intermediate dose level between DL1 and DL2 , TBD: To be determined 202 - DL1 - 02 202 - EC1 - 01 202 - DL2 - 02 203 - DL1 - 01 203 - DL1 - 03 203 - DL2 - 01 201 - DL1 - 01 202 - DL1 - 01 202 - DL1 - 02 202 - EC1 - 01 202 - DL2 - 01 202 - DL2 - 02 203 - DL1 - 01 203 - DL1 - 02 203 - DL1 - 03 203 - DL2 - 01 Data cut - off – February 16, 2021 ACT

ACTengine® Clinical Programs – Best Overall Response (BOR) Assessment Disease Control in 9 out of 10 Patients at Dose Level 1 and 2 (below 1 Billion Transduced CD8 T cells) IMA201 IMA202 IMA203 Patient 201 - DL1 - 01 202 - DL1 - 01 202 - DL1 - 02 202 - EC1 - 01 202 - DL2 - 01 202 - DL2 - 02 203 - DL1 - 01 203 - DL1 - 02 203 - DL1 - 03 203 - DL2 - 01 Patient ID 201 - 61 - 237 202 - 60 - 024 202 - 60 - 028 202 - 10 - 008 202 - 63 - 007 202 - 13 - 018 203 - 60 - 010 203 - 60 - 032 203 - 60 - 041 203 - 65 - 003 Dose level DL1 DL1 DL1 EC1 DL2 DL2 DL1 DL1 DL1 DL2 Total transduced cells 1 0.11x10 9 0.11x10 9 0.09x10 9 0.19x10 9 0.51x10 9 0.65x10 9 0.12x10 9 0.11x10 9 0.08x10 9 0.35x10 9 Age (gender) 60 (M) 33 (M) 63 (F) 64 (F) 68 (F) 49 (M) 40 (F) 63 (M) 61 (F) 57 (M) Diagnosis NSCLC HNSCC Squamous Cell Cancer Melanoma Squamous Cell Cancer Melanoma Head and Neck Cancer Ovarian Cancer Synovial Sarcoma Prior lines of systemic therapy 4 5 6 4 3 7 6 4 7 2 Prior lines of ICI 2 treatment 1 3 1 2 1 3 2 - 1 - Disease status at infusion Patients wi t h r ecurrent and/or refractory solid tumors Best response RECIST1.1 SD SD SD SD SD PD SD SD SD PR 3 15 1 Total infused dose of transduced viable CD8 T cells; 2 Immune checkpoint inhibitor; 3 Unconfirmed as of data cut - off; DL: Dose level, EC1: Enrichment cohort with intermediate dose level between DL1 and DL2 , SD: stable disease, PD, progressive di sease, PR: partial response Data cut - off – February 16, 2021 ACT 15

ACTengine ® Clinical Programs – Change of Sum of Diameters in Target Lesions Tumor Shrinkage Observed in 8 of 10 Patients at Low Dose Levels 16 1 Shortest diameter for nodal lesions; 2 Stable target lesions with parallel growth of a CNS non - target lesion ; 3 RECIST1.1 response at timepoint of maximum in change of target lesions (week 12): PD due to growth of non - target lesion; 4 PR unconfirmed as of data cut - off SD Best Overall Response (RECIST1.1) : PR PD 1 2 0 2 - 6 0 - 0 2 8 ( D L 1 ) 2 0 2 - 1 3 - 0 1 8 ( D L 2 ) 2 0 3 - 6 0 - 0 4 1 ( D L 1 ) 2 0 3 - 6 0 - 0 1 0 ( D L 1 ) 2 0 1 - 6 1 - 2 3 7 ( D L 1 ) 2 0 2 - 6 3 - 0 0 7 ( D L 2 ) 2 0 3 - 6 0 - 0 3 2 ( D L 1 ) 2 0 2 - 6 0 - 0 2 4 ( D L 1 ) 2 0 2 - 1 0 - 0 0 8 ( E C ) 2 0 3 - 6 5 - 0 0 3 ( D L 2 ) -40 -20 0 20 -39.9 -35.4 -16.2 -13.1 -11.1 -10.8 -9.7 -6.9 1.9 12.8 Change in Sum of Longest Diameter from Baseline [%] SD PD PR 202 - DL1 - 02 202 - DL2 - 02 2 203 - DL1 - 03 203 - DL1 - 01 201 - DL1 - 01 202 - DL2 - 01 203 - DL1 - 02 202 - DL1 - 01 202 - EC1 - 01 3 203 - DL2 - 01 4 Diagnosis S CC Melanoma Ovarian Cancer Head & Neck NSCLC SCC Head & Neck HNSCC Melanoma Synovial Sarcoma Data cut - off – February 16, 2021 ACT

ACTengine ® IMA204 – Targeting Tumor Stroma Complete Tumor Eradication in vitro & in vivo 1 by Affinity - enhanced IMA204 TCR CD8 - independent TCR leads to tumor eradication in all mice treated Control IMA204 TCR D7 D16 D22 D29 1 In vivo data by Jim Riley, University of Pennsylvania, control: non - transduced T cells. TCR avidity and specificity data not shown, avai lable in IMA204 presentation on Immatics website. 17 COL6A3 exon 6 prevalently expressed at high target density in tumor stroma across many solid cancers • CD8 - independent, next - generation TCR activates CD8 and CD4 T cells • Final preclinical safety evaluation ongoing, IMA204 clinical trial application expected 2021 Stroma cells Tumor cells Stroma Target (COL6A3 exon 6) in Ovarian Cancer sample Example of a Tumor Target in same Ovarian Cancer sample ACT

Combating Tumor Heterogeneity & Escape through Multi - Target Approach A Multi - Step Approach towards Highly Personalized Multi - TCR - T Therapy 18 ACTolog ® ACTengine ® TCR - T Personalized Multi - TCR - T 1 2 3 Combination Trial ACTengine ® Multi - TCR - T 4 >200 prioritized XPRESIDENT® targets Immatics TCR Warehouse Mission to treat every patient ACTolog® headline data presented at annual SITC conference available on the Immatics website HLA Targets T cells Status Objective 1 HLA - A2 Multiple Endogenous Completed Demonstrate feasibility of multi - target concept 2 HLA - A2 Single Genetically engineered 3 trials ongoing Deliver significant clinical benefit for patients with certain tumor types 3 HLA - A2 Two Genetically engineered Mid - Term Perspective Expand spectrum of tumor types and increase response durability 4 Multiple Multiple Genetically engineered Long - Term Perspective Treat every patient regardless of tumor and HLA type 1 2 3 4 Initial Warehouse ACT

ACTengine® IMA200 Series – Summary and Future Directions • IMA201, IMA202, IMA203 clinical trials • Complete Dose Escalation • Initiate Dose Expansion and treat patients at target dose • Update on patients treated at target dose expected for 2H2021 Next Steps 19 Transient and manageable treatment - emergent adverse events as expected for cell therapies Key Findings Robust T cell engraftment and persistence post infusion and tumor infiltration in all evaluable patients Tumor shrinkage observed in 8/10 patients including one unconfirmed partial response • IMA204 clinical trial application in 2H2021 First Anti - tumor A ctivity C onsistent with Robust Biological Activity during E arly Phases of Dose Escalation IMA204: Preclinical data: In vivo tumor eradication by targeting the tumor stroma with high - affinity TCRs • Preparation of first multi - TCR - T study ACT ACTengine® programs are supported by a grant of the Cancer Prevention & Research Institute of Texas (CPRIT)

ACTallo® IMA301 – Towards Off - the - shelf ACT Effective Redirection of γδ T cells Using αβ TCR 20 • Proprietary manufacturing protocol delivering robust expansion of γδ T cells with the potential for hundreds of doses from one single donor leukapheresis • Off - the - shelf cell therapy , applicable without need for personalized manufacturing and not reliant on potentially encumbered immune system of patient • High potency: TCR transduced γδ T cells show similar anti - tumor activity to αβ T cells • Proprietary single lentiviral vector system (4 - in - 1 construct) including TCR and CD8 alpha & beta chains • γδ T cells are abundant, show intrinsic anti - tumor activity, naturally infiltrate solid tumors and do not cause graft - vs - host disease γδ T cell collection from healthy donor Off - the - shelf product Transduction Expansion ACTallo ® Immatics’ Allogeneic ACT Approach ACT

TCR Bispecifics 21

TCER® – Immatics ’ TCR Bispecifics Off - the - shelf Biologics Linking Immune Cells to Tumor Cells 22 TCER®

TCER® – Superior Proprietary TCR Bispecific Format 23 TCER® 1 Based on comparative preclinical testing Maximizing efficacy x Selection of cancer peptide targets with unusually high t arget density (peptide copy number) x Individual maturation TCR CDR regions leading to >1000x enhanced affinity x Stability & Potency Optimized Format superior to six alternative Bispecific formats 1 leading to tumor eradication preclinically and targeting favorable treatment regimen in clinical trials (expected terminal half life: 1 - 2 weeks) Minimizing toxicity x Retention of high TCR specificity following affinity maturation by XPRESIDENT® - guided similar peptide counterselection x Optimized Affinity of T cell engager vs. TCR targeting compound enrichment in tumor TCER® T cell recruiting antibody pHLA targeting TCR Fc domain (silenced) with KiH technology

TCER® IMA401 Targeting MAGEA4/8 High Specificity, Potency in Animal Models and Favorable Half - life 24 * TCER® Study day - 14: transplantation of tumor cells Study day 1: human PBMC transplantation & start of IMA401 weekly treatment Patient - Derived Tumor Model 2 Preclinical Proof - of - Concept Data: • High affinity TCR (2 nM ) after >10,000 - fold affinity - maturation via yeast display • High potency at low concentrations in vitro and in vivo in two independent xenograft tumor models (NSCLC and melanoma) 1 • Distinguished specificity & broad therapeutic window (≥ 1,000 - fold concentration difference between tumor vs. healthy cell reactivity) • Favorable CMC characteristics and pharmacokinetics with 10 - 11 days terminal half - life in mice Development Status • GMP production of clinical batch completed with high production yield • Positive feedback on trial design, preclinical safety and efficacy package from regulators in scientific advice meetings • Clinical Trial Application on track for 4Q 2021 1 Patient - derived LXFA 1012 (NSCLC, adenocarcinoma) tumor xenograft model in NOG mice; data not shown from cell line - derived Hs695T (melanoma cell line) tumor xenograft model in NOG mice ®

TCER® IMA401 Targeting MAGEA4/8 High Target Density Across Multiple Tumor Indications >5 - fold higher target density 1 than a commonly used MAGEA4 target peptide 25 MAGEA4/8 Peptide ( q uantitative mass spectrometry detection) Normal tissue Cancer tissue Status as of Oct 2020 MAGEA4/8 target peptide is naturally and specifically presented on native tumor tissue vs. various normal tissues 1 Copy number per tumor cell ( CpC ) measured on a paired - sample basis by AbsQuant ®, i.e. comparing MAGEA4 vs. MAGEA4/A8 peptide presentation on same sample, 2 Students paired T test TCER® p<0.001 2

TCER® IMA402 Targeting PRAME High Specificity and Anti - tumor Activity in vitro and in Mice Studies 26 TCER® Preclinical Proof - of - Concept Data: • High - affinity TCR Candidates after affinity - maturation via yeast display • High potency at low concentrations and physiological target density in vitro and in vivo in xenograft tumor model (data not shown) • Distinguished specificity & broad therapeutic window (≥ 1,000 - fold concentration difference between tumor vs. healthy cell reactivity, exemplary data) Development Status • Service Agreement with CDMO signed & manufacturing activities started 0 25 50 75 100 125 150 175 200 10 -1 10 0 10 1 10 2 10 3 10 4 10 5 TCER [pM] % L y s i s w/o iPSC - derived Cardiomyocytes Tumor cell line (~500 target pHLA per cell ) Similar data available across different (n=11) human normal tissues IMA402 candidate

TCER® IMA402 Targeting PRAME High Prevalence and Homogeneity of PRAME across Multiple Tumor Indications 27 Normal tissue Cancer tissue PRAME Peptide detection (MS) SqNSCLC Ovarian Cancer PRAME RNA detection in tumor samples (ISH) Indications Target p revalence [%] Uterine carcinoma 100 Melanoma 95 Ovarian carcinoma 80 Squamous non - small cell lung carcinoma 65 Uveal melanoma 50 Cholangiocarcinoma 35 Diffuse large B - cell lymphoma 30 Breast carcinoma 25 Head & neck squamous cell carcinoma 25 plus several further indications PRAME target prevalence in selected cancer indications PRAME target prevalences are based on TCGA data combined with a XPRESIDENT® - determined target individual MS - based mRNA expression threshold TCER®

Discovery Platforms 28

XPRESIDENT® – Discovery of True Cancer Targets Pool of 200 Targets as Foundation for our Future Pipeline >2,500 cancer & normal tissues a nalyzed by Quantitative, Ultra - Sensitive Mass Spectrometry pHLA Database based on primary tissues >200 prioritized targets Technology 29 200 Prioritized Targets Grouped in 3 Target Classes: Unbiased Identification of the most relevant pHLA targets in the accessible cancer immunopeptidome 1. Well known and characterized parent protein e.g. MAGE family cancer testis antigens 2. Unknown or poorly characterized parent protein e.g. stroma target COL6A3 exon 6 3. Crypto - targets/Neoantigens: Novel target class which includes RNA - edited peptides & non - classical neoantigens 3. Crypto 2. Unknown / less known 1. Well Known

Development of the Right TCR – XCEPTOR® Unique Cross - Talk between Target and TCR Discovery 30 TCR Bispecifics T cell engaging receptor (TCER®) Affinity - maturated natural TCR variable domains w ith nanomolar affinity and favorable specificity profile XPRESIDENT® - guided similar peptide counterselection during maturation to deselect cross - reactive TCRs Basis for h ighly potent TCR Bispecifics format Natural or optimized natural TCR with micromolar affinity and favorable specificity profile for genetic engineering of T cells and direct clinical application TCR Discovery, Engineering and Validation Fast and efficient discovery of multiple TCRs per target XPRESIDENT® - guided off - target toxicity screening t o de select cross - reactive TCRs during discovery Adoptive Cell Therapy ACTengine® ACTallo ® Technology

Corporate Information & Milestones 31

Robust IP Portfolio Immatics’ Patent Estate – Territorial Coverage 32 • >8,000 cancer targets, TCRs and technology protected by • 3,500 applications and patents filed in all major countries and regions • >100 patent families • >1,550 granted patents, thereof >400 granted patents in the US Corporate

Strong, Focused and Highly Integrated Trans - Atlantic Organization 33 Senior Leadership, Business Development, Clinical Operations, Intellectual Property, Regulatory Affairs, Communications Senior Leadership, Research and Development (Adoptive Cell Therapy), CMC, Clinical Operations, Regulatory Affairs, QA/QC, HR, Investor Relations Munich, Germany, ~40 FTEs Tübingen, Germany, ~160 FTEs Houston, Texas , ~ 10 0 FTEs Senior Leadership, Research and Development (XPRESIDENT®, XCEPTOR®, TCER®), Translational Development, Clinical Operations, Finance, HR, IT, QM Corporate FTE status as of 30 June 2021

Experienced Global Leadership Team Across Europe and the US 34 Harpreet Singh Chief Executive Officer Co - Founder 20 yrs biotech experience Carsten Reinhardt Chief Development Officer >20 yrs pharma & biotech experience ( Micromet , Roche, Fresenius) Rainer Kramer Chief Business Officer 25 yrs pharma & biotech experience (Amgen, MorphoSys , Jerini , Shire, Signature Dx) Steffen Walter Chief Technology Officer Co - Founder Immatics US >15 yrs biotech experience Arnd Christ Chief Financial Officer 20 yrs biotech experience ( Probiodrug , NovImmune , Medigene , InflaRx ) Toni Weinschenk Chief Innovation Officer Co - Founder > 15 yrs biotech experience Jordan Silverstein Head of Strategy 10 yrs biotech experience (Advanced Accelerator Applications, InflaRx ) Edward Sturchio General Counsel >15 yrs pharma & biotech experience (Schering, Merck, Novartis, Advanced Accelerator Applications, Abeona Therapeutics) Cedrik Britten Chief Medical Officer >10 yrs pharma & biotech experience ( BioNTech , GSK) Corporate

Upcoming R&D Milestones in 2021 35 1H 2021 2H 2021 ACTengine ® IMA201, 202, 203: Initial dose escalation read - out IMA201: Additional read - out at dose level 1 IMA204: IND * submission TCER® IMA401: IND * submission IMA202: Additional read - out at dose level 2 and 3 IMA402: Preclinical PoC & start GMP mf. activities * IND: May be investigational drug application with FDA or analogous clinical trial application (CTA) to a European regulatory age ncy Corporate IMA203: Additional read - out at dose level 2 and 3

Immatics Key Take - Aways 36 • Broadly positioned in TCR therapeutics space with two distinct treatment modalities: ACT & TCR Bispecifics • ACTengine ® (TCR - T) IMA200 Clinical Series • Proprietary cell manufacturing resulting in younger T cells for better engraftment & persistence • First anti - tumor activity observed in three TCR - T trials at early phases of dose escalation – next readout in 2H21 • TCER® - Leading TCR Bispecifics platform with antibody - like stability and half - life • Clinical trial application on track in 4Q21 for IMA401 program against high density target • Preclinical proof - of - concept demonstrated for IMA402 against highly prevalent target PRAME • Differentiated target and TCR discovery platforms secured by a broad patent estate including >200 prioritized targets • Multiple strategic collaborations with world - leading industry players incl. Amgen, Genmab, BMS and GSK • Strong cash position of approx. US$ 254m (as of March 30, 2020) with cash reach into 2023 Corporate

www.immatics.com Thank you
